Explain the Difference Between Metals and Nonmetals
Non-metals are brittle and could be broken down into pieces. Some of them dissolve to form alkali.
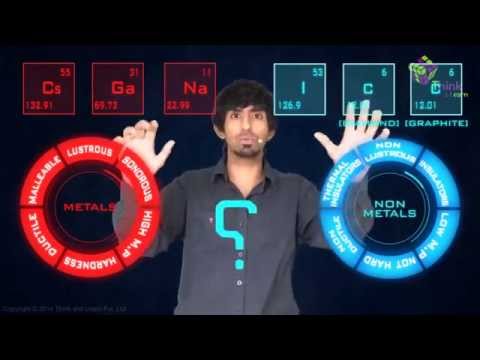
Difference Between Metals And Nonmetals Definition Classifications Differentiation Examples With Videos And Faqs Of Metal And Nonmetals
Metals are good conductors of heat and electricity.

. Learn vocabulary terms and more with flashcards games. Formation The volume of air that you calculate for a 30-minute dive was the volume of air breathed on land. Metals have a certain luster or shine while non-metals are dull.
At room temperature metals are usually solid except mercury and gallium which are in the liquid state. 4 it can lose the electron. Metals react with oxygen to form basic oxidesZn and Al form amphoteric oxides which show the properties of both acidic and basic oxidesMostly metal oxides are insoluble in water.
Metals have a high reducing power. Another difference is that metals tend to lose their valence electrons but non-metals share or gain valence electrons. Metals form oxides that are basic but non-metals form oxides that are acidic.
5 all metals are in the solid state at room temperature except mercury which is in the liquid state. Have properties of both metals and non-metals. For example sulfur and carbon are both non-metals.
Metals are the elements which exhibit the highest degree of metallic behavior is known as metals on the contrary Non-metals are such elements which do not possess any metallic behavior and Metalloids are those elements that possess some of the properties like metal while some like. Metals are electropositive in nature. Chemical properties Nature of oxides Metallic oxides are basic in nature.
Metals loose electrons to form electropositive ions whereas non-metals accept electrons to form electronegative ions. Non metals are generally brittle and can break down into smaller pieces. They do not allow heat and electricity to pass through them.
Explain how the differences in valence electrons between metals and nonmetals lead to differences in charge and the giving or taking of electrons ion. Properties of metals can be explained in terms of metallic structure and bonding. They can be beaten into sheets and wires.
Metals and non-metals have different properties. Metals have one to three electrons in their outer shell whereas non-metals have four to eight electrons. For example non metals have lower melting and boiling points as compared to metals.
Conduction They conduct heat and electricity. Nonmetals have high ionization enthalpies. Different chemical models have.
6 rows Metals are lustrous and can be polished. Non-metals are non-lustrous dull and cannot be. Metals have low ionization enthalpies.
1 shiny in nature lustrous 2 good conductor of heat and electricity. Metals are found in the left side of the periodic table whereas non-metals are found on the right side of the periodic table. Metals are sonorous they produce a ringing sound on beating.
Non metals are soft except for diamonds. Nonmetals however come in different colors. 1 poor conductor of heat and electricity except graphite.
Metals when subjected to chemical changes loose electrons while nonmetals gain electrons and turn into anions. Metals generally form ionic compounds. Although they are all chemical elements they have different chemical structures and most of their attributes would be noticeably opposites.
Metals are elements having the highest degree of metallic behavior. Key Differences Between Metals Non-Metals and Metalloids. Reaction with water Metals react with water.
They are shiny and lustrous. Metals are generally malleable and ductile. They are rather electropositive elements.
Oxides of non-metals are acidic. Metals and non metals have the most different physical and chemical characteristics. Difference Between Metals Nonmetals and Metalloids Definition.
Block in the. They are not shiny and are non lustrous except iodine. Start studying The difference between metal elements and non-metal elements.
They are electropositive in nature. Except for diamond these non-metals are soft. Brittle lack luster cannot conduct electricity.
Except for sodium these metals are very hard. Metals are less electronegative. Nonmetals generally form covalent compounds.
Position in the Periodic Table. For example sulfur and carbon are both non-metals. Non-metals do not have properties present in metals whereas metalloids are elements that have intermediate properties of both metals and non-metals.
The non-metals exist in all three states. The main difference between metals non-metals and metalloids are that metals are elements that are hard malleable fusible shiny ductile and good conductors. Where metals form oxides which are basic non-metals form oxides which are acidic.
Metals are ductile and malleable. Conversely non-metals can be found in solid or gaseous form except Bromine which is the only non-metal that is present in liquid form. Non-Metals are Electronegative in nature.
Metals generally form basic oxides while nonmetals are good oxidizing agents. Non-metals react with oxygen to form oxidesNon- metal oxides are soluble in water. Nonmetals have a low reducing power.
Metals are found in the left side of the periodic table. If both elements were metals or if both were nonmetals the electronegativities would be too similar for one element to take electrons from the other. Metals have one to three electrons in their outer shell whereas non-metals have four to eight electrons.
Metals have a crystalline structure whereas non-metals possess amorphic structure. The difference in electronegativity between metals and nonmetals is high meaning that its very easy for the very electronegative nonmetals to take electrons from non-electronegative metals. They are electronegative in nature.
3 high melting point And the density.

Write Differences Between Metals And Non Metals Based On Physical Properties Brainly In

Difference Between Metal Nonmetal Hindi Youtube

Write The Difference Between Metals And Non Metals Under The Following Heads I Nature Ii State Iii Lustre Iv Ductility V Malleability Vi Conductivity
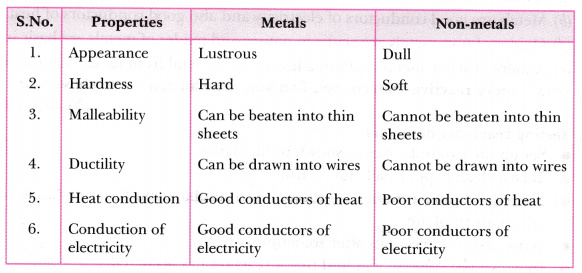
Distinguish Between Metals And Non Metals On The Basis Of The Following Properties Appearance Hardness Malleability Ductility Heat Conduction Conduction Of Electricity Cbse Class 8 Science Learn Cbse Forum

What Are The Major Differences Between Metals And Non Metals Target Batch

What Is The Difference Between Metal Aur Non Metals Brainly In

Metals Metalloids Nonmetals Share Some Properties Of Metals And Some Of Nonmetals Some Are Shiny Som Chemistry Lessons Chemistry Education Chemistry Classroom

List Three Difference Between Metals And Non Metals Brainly In
Difference Between Metals Metalloids And Nonmetals Difference Between
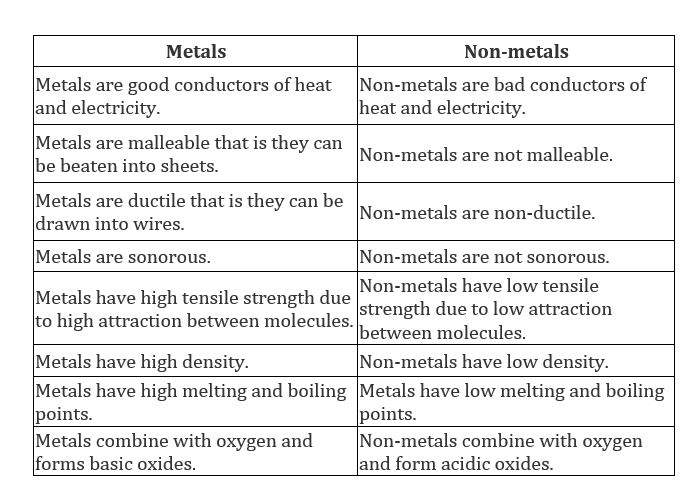
Difference Between Metal And Non Metal Chemistry Topperlearning Com 31bnk8rr

Cbse Class 10 Chemistry Differentiate Between Metal And Non Metal On The Basis Of Their Chemical Properties
Difference Between Metals Nonmetals And Metalloids Definition Properties Examples

Write The Difference Between Metal And Non Metal On The Loos Is Of Physical Properties Snapsolve
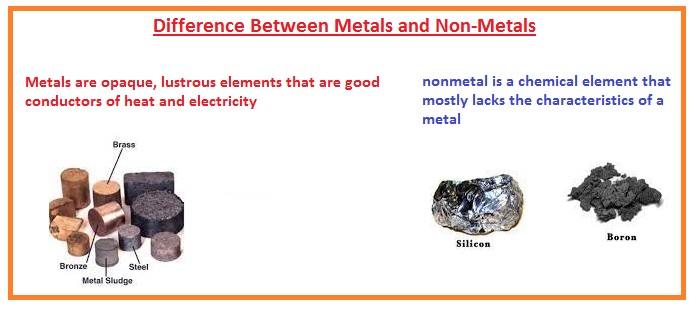
Difference Between Metals And Non Metals The Engineering Knowledge

List Three Differences Between Metals And Non Metals Snapsolve
What Is The Difference Between Metal And Nonmetal Quora

Differentiate Between Metal And Non Metal On The Basis Of Their Chemical Properties

Difference Between Metals And Non Metals Metals Vs Non Metals

Distinguish Between Metals And Non Metals What Are Matalloids Give Any Two Examples Of Metalloids Brainly In
Comments
Post a Comment